In addition offering free consultations and fee-for-service laboratory services, the Center has developed a Partnership Program to facilitate the development of new therapies at the University.
This program utilizes funds allocated exclusively for the purpose of translating discoveries by University of Minnesota investigators into the clinic. The Center for Translational Medicine (CTM) works with inventor(s) to create a development plan and utilizes these funds to conduct the preclinical testing and manufacturing necessary to translate the discovery into the clinic. The Center also supports IND/IDE development, preparation of clinical supplies, and Phase I clinical trial design and implementation.
Approval process and selection criteria
Applications are accepted on a rolling basis. The completed application will be submitted to the Scientific Advisory Board for review and approval. Selection criteria include: scientific merit, developmental feasibility, clinical impact, resource and expertise requirements, regulatory pathway and current or potential funding sources.
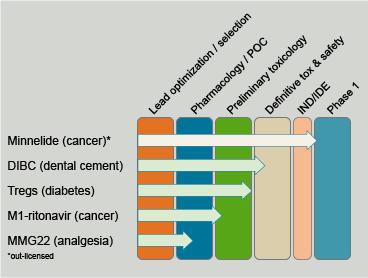
The Center for Translational Medicine has partnered with inventors from across the University to develop drugs, biologics, devices and combination therapies. Current Partnership Programs include potential therapies for diabetes, cancer and pain (see graphic).
Contact the scientific director, Robert J. Schumacher, PhD, for more details about the Partnership Program or for more information about how the CTM supports translational development.